As described by the CDC, “Infectious diseases whose incidence in humans has increased in the past 2 decades or threatens to increase in the near future have been defined as “emerging.” These diseases, which respect no national boundaries, include:
Central nervous system infections cause significant mortality and morbidity worldwide, but especially in low- and middle-income countries. According to the World Health Organization, Central nervous system infections caused ~35 million Disability Adjusted Life Years (DALYs) globally in 2012. Clinical outcomes are highly dependent upon the rapid identification of the causative agent and instituting effective (antimicrobial) therapy. However, current diagnostics are inadequate and the causative agent is identified in <50% of the patients. Furthermore, Southeast Asia (including Vietnam) is highly susceptible to vector-borne diseases (e.g. Japanese encephalitis and Zika virus), vaccine-preventable diseases (e.g. Streptococcus pneumoniae), multi-drug resistant organisms (e.g. Mycobacterium tuberculosis) and emerging novel pathogens (e.g. Nipah virus). New diagnostic approaches are urgently required for the rapid response to these evolving challenges and to improve patient outcomes.
We aim to answer three main research questions:
Despite extensive diagnostic efforts the majority of severe infections (as sepsis and acute central nervous system infections) remains undiagnosed in routine clinical situations, but also in clinical studies with the aim to detect all known pathogens. For example, in clinical studies carried out by OUCRU in Viet Nam, between 20-40% of respiratory infections and 40-60% of central nervous system infections a pathogen could not be detected. This implies that there are still pathogens causing disease that we are unaware of, while South East Asia (including Vietnam) is predicted to be one of the hotspots of future emerging infections. Knowledge of which pathogens cause these severe diseases and building of local capacity that is capable of dealing with future emerging infections of unknown origins are important for both clinical management and public health interventions.
Our pathogen discovery programme aims to:
Hand, foot and mouth disease is an emerging infection of major public health significance in the Asia-Pacific region. Annually, hand, foot and mouth disease affects over two million children in Asia. In 2011 and 2012 Vietnam experienced two large outbreaks with over 200,000 children requiring hospitalization and over 200 deaths. Currently, there is no effective antiviral drug available to treat the infected patients, while vaccine has only been available for enterovirus A71, but its use is limited within China.
Some of the key questions we are trying to answer with our current clinical and laboratory research program on hand, foot and mouth disease:
Clinical research, whether observational or interventional, has become an intrinsically slow process and the time from a completed protocol to the first enrolled patient could be up to two years for several reasons. This has been a major barrier to initiating clinical research in outbreak settings as was demonstrated during the 2009 pandemic of influenza (H1N1-pdm09). To address the need for change in our approach to research in these situations, OUCRU is collaborating with ISARIC, the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC).
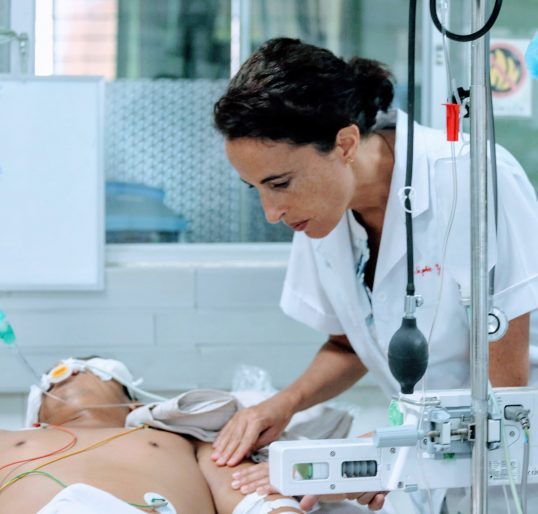
Despite being a vaccine preventable disease, tetanus continues to be a problem in Vietnam. Tetanus is a severe disease where muscle spasm affects the whole body, eventually interfering with the ability to breath. In many countries, 50% or more people contracting tetanus will die because of this. Patients with tetanus need treatment in the intensive care unit for several weeks at least which also exposes them to other dangers such as hospital acquired infection with multidrug resistant organisms. We have been working with the Hospital for Tropical for Tropical Diseases on ways of improving tetanus treatment for over 20 years. We are working both on treating the disease better but also preventing it occurring. Our main questions are
Vietnam ICU Translational Applications Laboratory (VITAL) is a unique multidisciplinary project, funded by Wellcome as part of its Innovations for Impact strategy, providing ICU/critical care innovation in resource-limited settings.