Age, literacy level, and health status shape participants’ understanding of clinical trials. In a recently published study, OUCRU researchers observed the consent process in two trials and noted many ways that participants’ understanding of study information can be enhanced.
Informed consent is an essential process when doing clinical trials involving human subjects, according to Good Clinical Practice. This step ensures that trial participants are aware of the trial information, study purpose, potential risks, and benefits to their health before making an informed decision to participate.
Studies at OUCRU follow international and national guidelines on ethical standards and procedures for biomedical research involving human subjects—however, a new social science study by Nguyen Thi Hong Yen et al. found participants still had many different perceptions and misunderstandings of research and clinical trial information.
The study was embedded in two randomised clinical trials conducted by OUCRU and Hospital for Tropical Diseases (Ho Chi Minh City). One was an outpatient clinical trial involving patients with chronic liver conditions. The other was an in-patient clinical trial involving patients with TB meningitis.
Both clinical trials did not involve high-risk interventions. However, some participants enrolled in the in-patient clinical trial were quite ill. Participants in the trials received drugs and examinations per trial protocol, as well as had travel costs reimbursed.
The following infographic describes the findings.




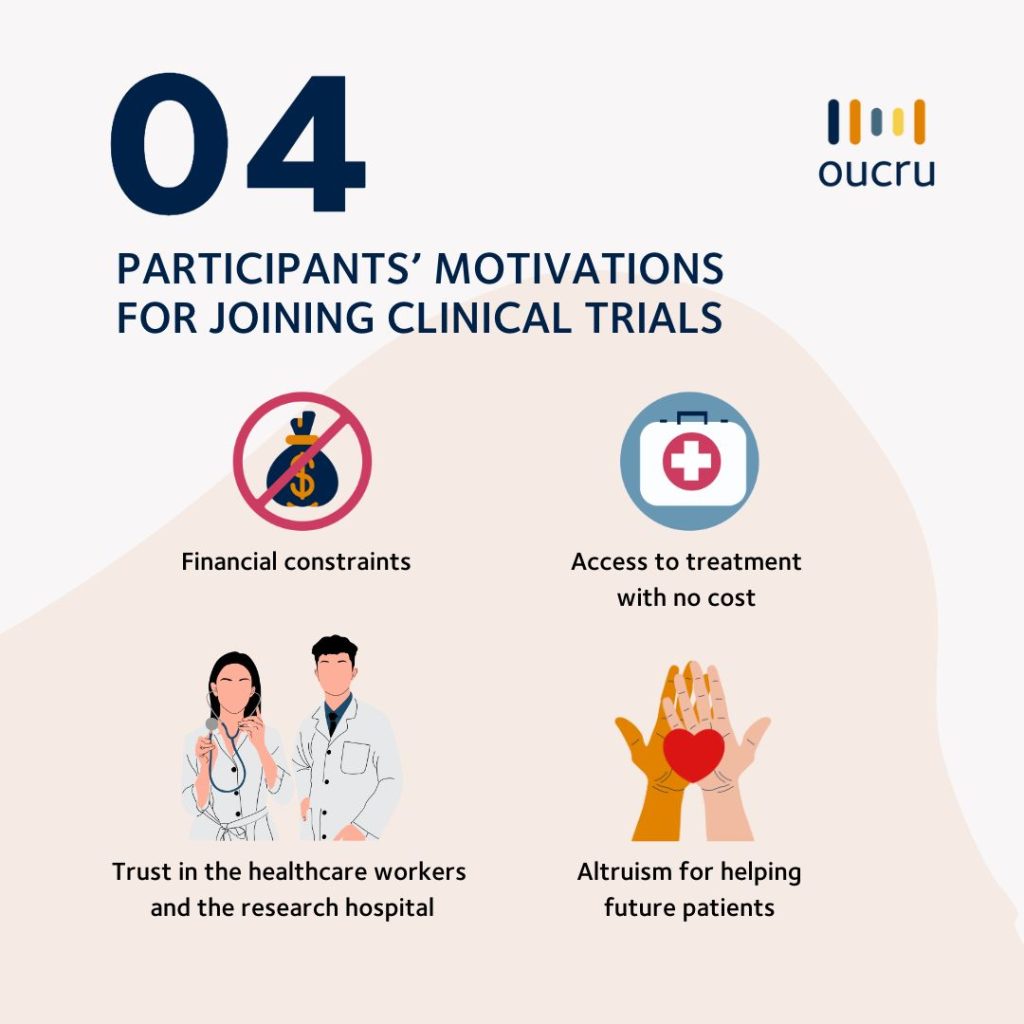
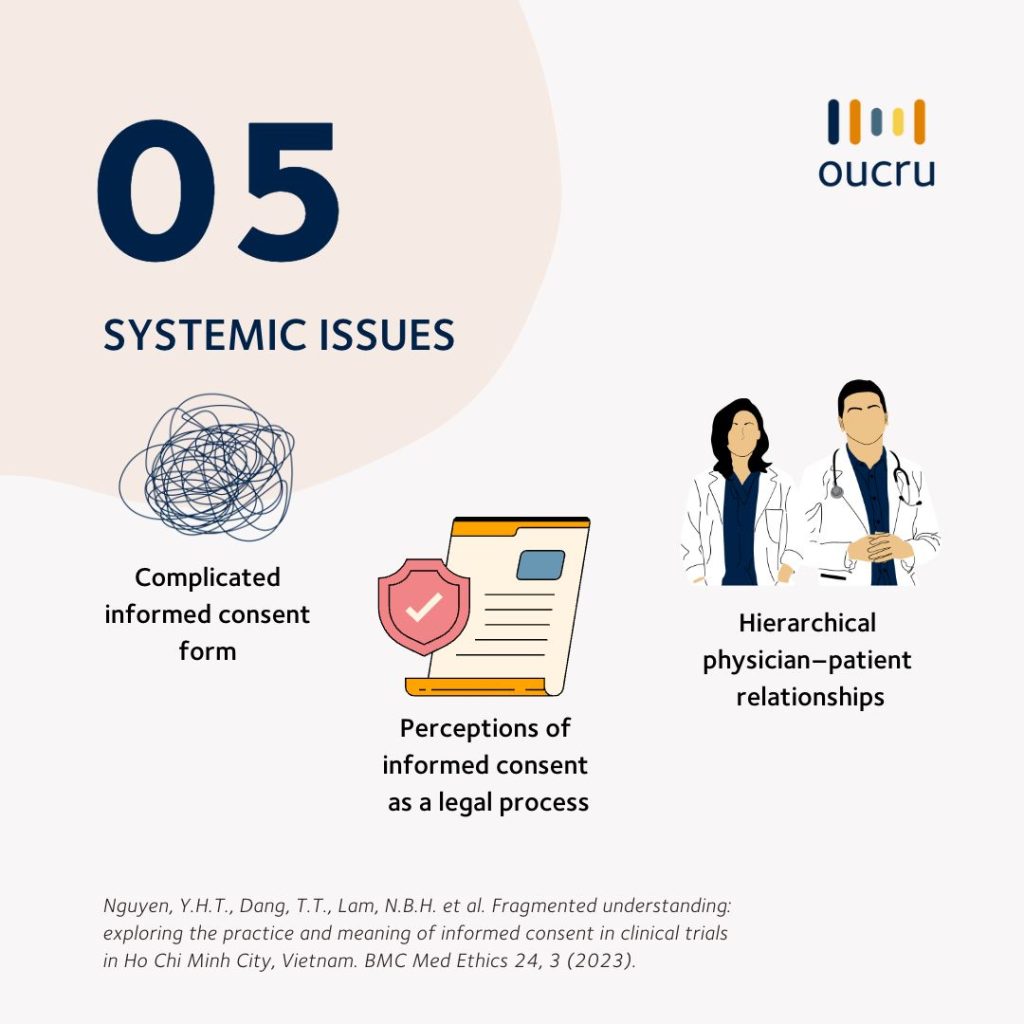
The research team stressed that “clinical trial teams and research institutions need to recognize their responsibility to improve the process and protect participants in research. Improved access to quality healthcare for poor patients can also reduce their vulnerabilities when making the decision to join clinical trials.”
The findings also pointed out ways clinical trials can improve the informed consent process to enhance participants’ understanding.
The first step we took was redesigning trial documents with pictures and larger font sizes and testing the design with our community advisory board. The board members found the revised forms improved readability and enhanced their understanding of the information.
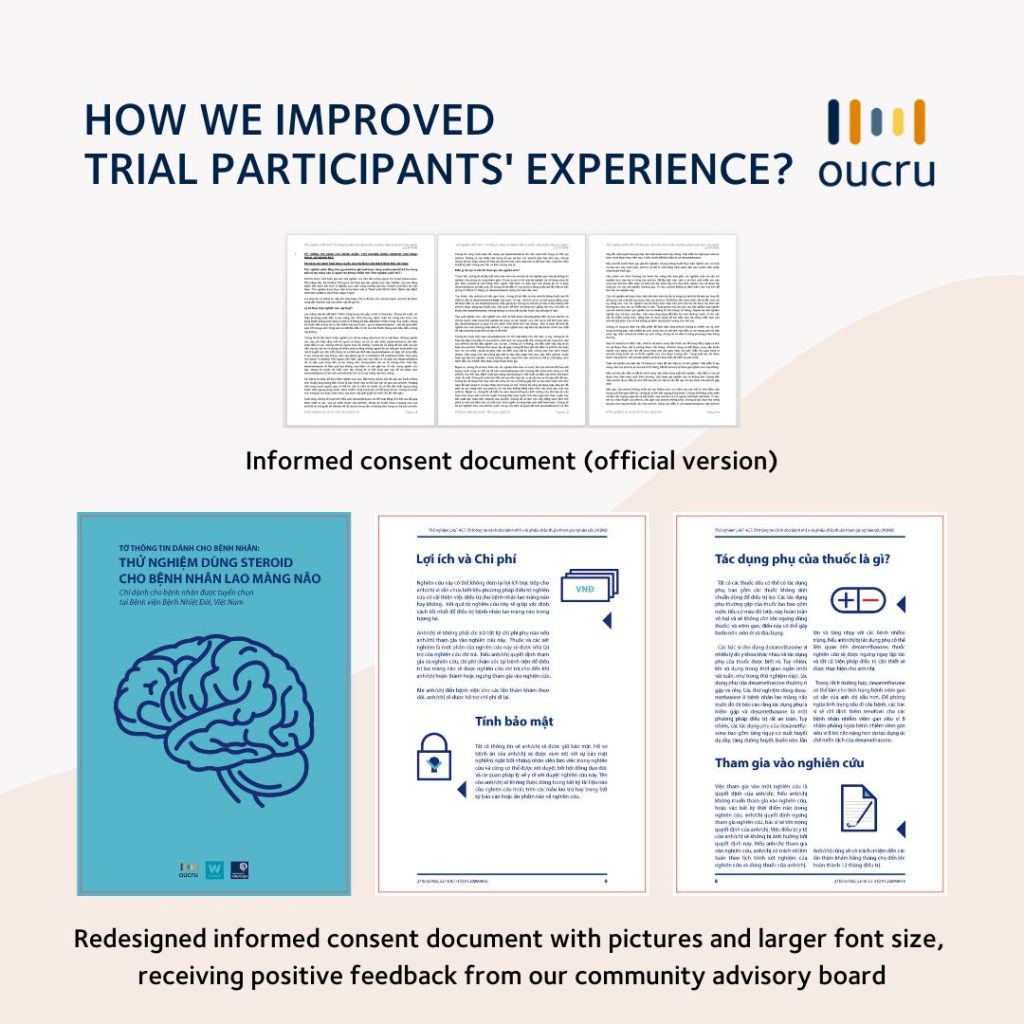
Similar studies also suggested other ways to improve participants’ understanding of the clinical trial. These methods include providing additional written information, using audiovisual and multimedia tools, extended discussions and test/feedback techniques, which improved the study participants’ comprehension and understanding of risks and procedures.
Citation
Nguyen, Y.H.T., Dang, T.T., Lam, N.B.H. et al. Fragmented understanding: exploring the practice and meaning of informed consent in clinical trials in Ho Chi Minh City, Vietnam. BMC Med Ethics 24, 3 (2023). https://doi.org/10.1186/s12910-023-00884-2











